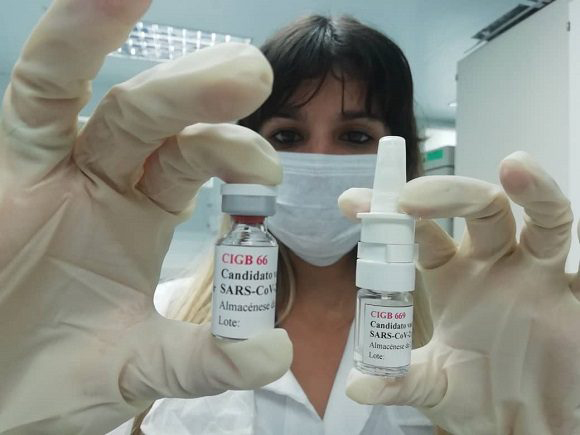
The Cuban Center for State Control of Medicines, Equipment and Medical Devices approved the phase I/II clinical trial to evaluate the safety and immunogenicity of the vaccine candidate Mambisa -intranasal- and the Abdala vaccine -intramuscular-, a study to boost immunity in convalescents of COVID-19.
Read more Cuba approves clinical trial with Mambisa and Abdala in COVID-19 convalescents
The province of Holguín has included new population groups to the anti-COVID-19 vaccination programs, as part of the extension of immunization among the priorities of the public health sector in Cuba.
Read more Holguín: New population groups incorporated to anti-COVID-19 vaccination programs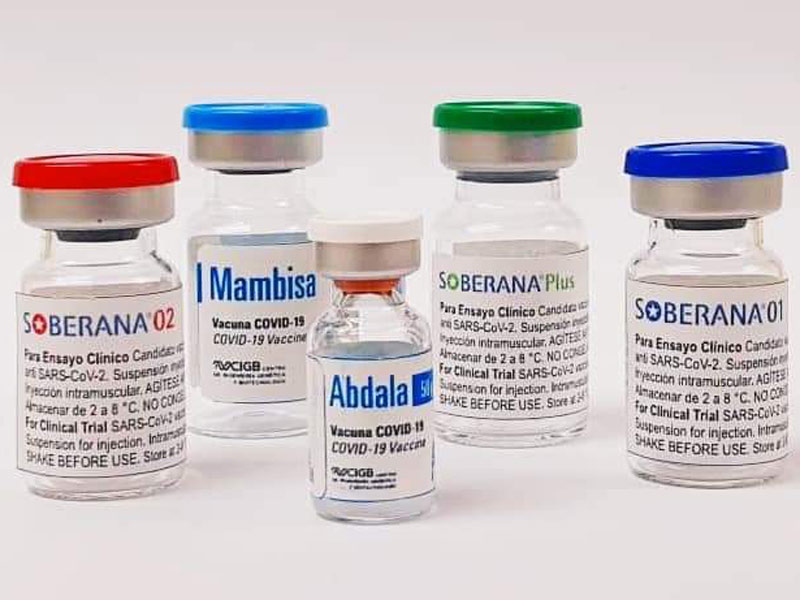 Cuba's Soberana Plus vaccine candidate against Covid-19 is currently showing encouraging results by considerably raising antibodies in Covid-19 convalescents with only one dose.
Cuba's Soberana Plus vaccine candidate against Covid-19 is currently showing encouraging results by considerably raising antibodies in Covid-19 convalescents with only one dose.

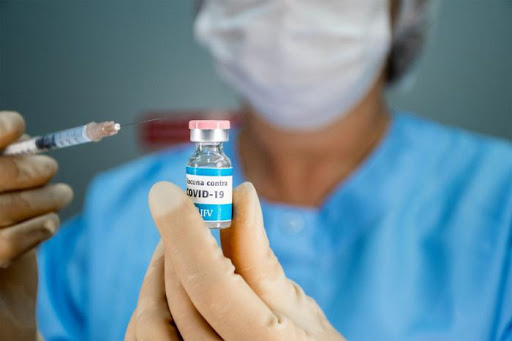
Cuba will start this month a broader intervention study with the vaccine candidates Abdala and Soberana 02, prioritizing complex territories and risk groups. Then, after the approval of the emergency use, the island will increase the scope of immunization, and by August, 70% of its population will be vaccinated.
Read more 70 % of the Cuban population will be vaccinated against COVID-19 by AugustMore Articles
Page 379 of 466